
Eversense® Continuous Glucose Monitor (CGM): A Post-Approval Study to Evaluate the Safety and Effectiveness of the Eversense® CGM System Used Non-Adjunctively (without another meter to compare)
Eversense® Continuous Glucose Monitor (CGM): A Post-Approval Study
Protocol Number: CTP-0039
Sponsor: Senseonics™
The system will consist of: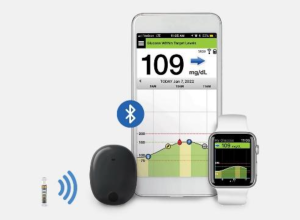
- Glucose Sensor, (approximately 3.5 mm x 18.3 mm length (Inserted under the skin in the triceps area of either arm)
- Battery-powered external Transmitter (Worn externally over sensor-placed with an adhesive patch) The transmitter vibrates to signal highs or lows.
- Mobile Medical Application (MMA) available on Android or iOS smartphone or smartwatch which will display glucose information.
Summary of Study Details:
The purpose of this study is to evaluate the safety and effectiveness of the Eversense 365 day CGM used non-adjunctively (without comparing it to another meter). You will go through a pre-approval process through your insurance company to determine your out-of-pocket cost, if any. You will receive a stipend which may help cover the out-of-pocket costs.
There are 3 study visits once the insurance approves the device and sends the device to either you or the office.
- The first visit, the 365 sensor is placed under the skin with a local anesthetic. The procedure takes approximately 15 minutes. The CGM is activated, and we discuss how to use the device. We also complete an A1c blood draw and questionnaire. When the transmitter is worn over the inserted sensor, participants will be able to see their blood sugars on their Eversense Mobile App. You will be given a glucometer and test strips to calibrate the CGM daily.
- There is a second visit about 6 months later to check an A1c and final questionnaires are administered.
- The final visit is on week 52 once the 365-day sensor has completed its cycle to remove the CGM. Another blood draw is done to check A1c and questionnaires are administered.
General Eligibility Criteria
- Must have a smart phone that can download the Eversense App and be able to use the App
- Must NOT have prior use of any Eversense CGM device.
- Must NOT have known contraindication to dexamethasone or dexamethasone acetate.
Compensation: You’ll receive a check for $150.00 at Visit 1, $300.00 at Visit 2 and $150.00 at Visit 3 for a total of $600 for the completed study, which may help cover any out-of-pocket costs for the device.
If interested in learning more, please contact our Research Coordinator, Jamie Sigg, RD, CDCES, CRC at (303) 321-2644 x 214 or email jsigg@denverendocenter.com
